Introduction
People may experience impaired decision-making due to a sudden illness or accident, progressive conditions such as dementia, or an intellectual or learning disability. Research involving adults who have impaired capacity to consent can raise ethical, legal, and practical issues.
Difficulties overcoming these issues mean that people who lack capacity are generally under-represented in research, resulting in a lack of evidence base for their care. Greater understanding about how we can appropriately involve them in research, whilst protecting their interests and welfare, is needed.
Our previous research has shown that knowing when and how to include adults with impaired capacity in research can be challenging in practice. This includes understanding the ethical and legal justification, how to design inclusive studies, who should be involved in making decisions about participation, what documents are required and what information they should contain.
Difficulties overcoming these issues mean that people who lack capacity are generally under-represented in research, resulting in a lack of evidence base for their care. Greater understanding about how we can appropriately involve them in research, whilst protecting their interests and welfare, is needed.
Our previous research has shown that knowing when and how to include adults with impaired capacity in research can be challenging in practice. This includes understanding the ethical and legal justification, how to design inclusive studies, who should be involved in making decisions about participation, what documents are required and what information they should contain.
Aim of these resources
These pages contain a range of information and resources to support the design and conduct of research involving adults with impaired capacity to consent that have been collated under a number of themed areas. The information and resources can be accessed by clicking on the theme headings below or using the menu bar. Brief summaries of the consent processes can also be found below.
Designing inclusive studiesResources on designing studies to be inclusive of adults with impaired capacity to consent, including legal frameworks, outcomes and data collection, and participatory approaches
|
Research
|
Communication and capacityResources to help ensure that communication about research is accessible, information about assessment of capacity, and supporting adults with impaired capacity to make decisions
|
Consent and
|
Research
|
Commonly used
|
Brief summaries of consent processes
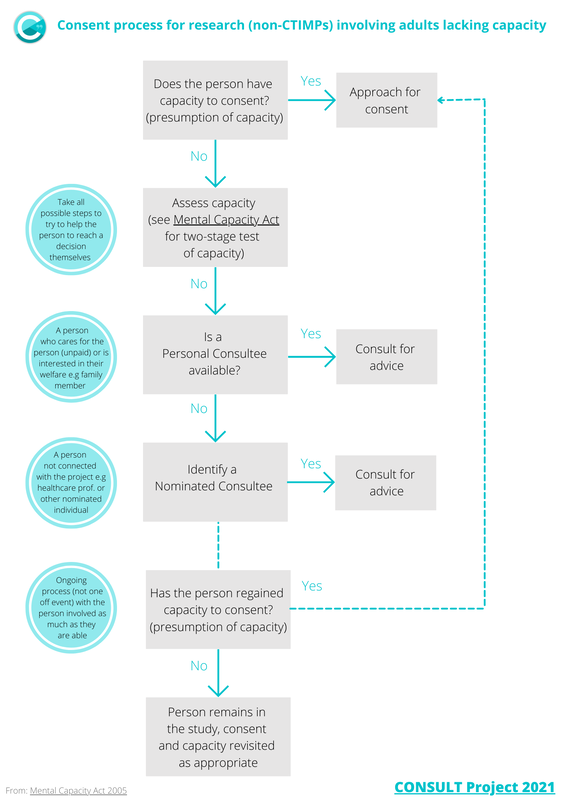
Non-CTIMPs involving adults lacking capacityBrief flow diagram of the consent process for intrusive research governed by the Mental Capacity Act 2005 in England and Wales
|
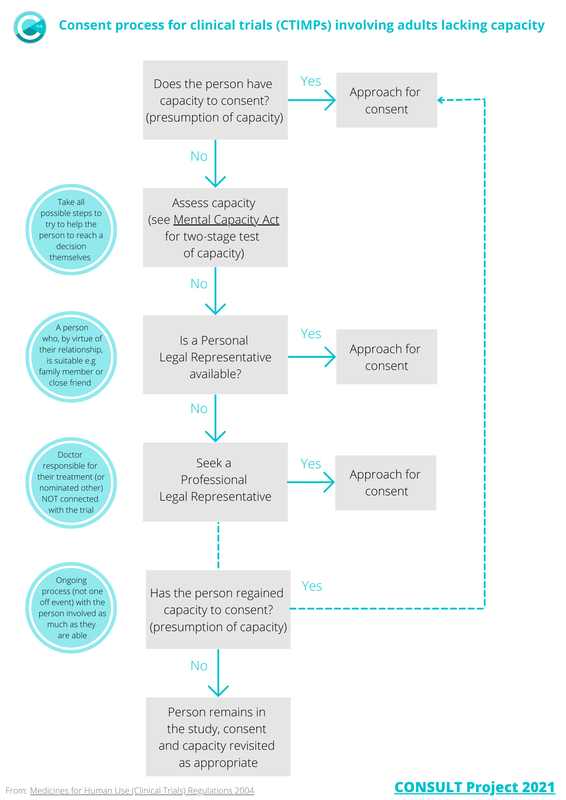
CTIMPs involving adults lacking capacityBrief flow diagram of the consent process for clinical trials governed by the Medicines for Human Use (Clinical Trials) Regulations 2004 in England and Wales
|
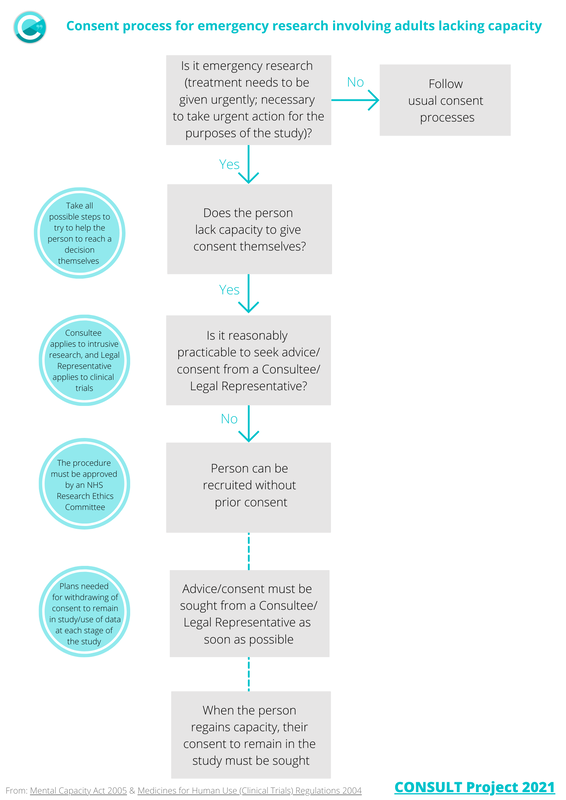
Emergency research involving adults lacking capacityBrief flow diagram of the process for emergency research (CTIMPs and non-CTIMPs) conducted without prior consent or consultation in England and Wales
|
Case studies
A randomised controlled trial with care home residents: the PRINCESS trial
RANDOMISED CONTROLLED TRIAL OF COMPUTERISED SPEECH AND LANGUAGE THERAPY FOR APHASIA: BIG CACTUS