Principles of consent
For consent to be considered both legal and ethical it must be:
Although most adults are able to make decisions for themselves (and there is a presumption under the Mental Capacity Act that adults are able to unless proven otherwise), there are some adults who will not have capacity to do so. The Communication and Capacity page of the website has more information on assessing capacity for research and how to ensure that the person is informed and supported in order to maximise their ability to their own decision where possible.
There are additional legal and ethical considerations for adults who lack capacity to be included in research. More information can be found on the Legal Frameworks page and Research Ethics page.
Consent is considered to be an iterative and on-going process rather than a one-off event. Researchers will need to consider what processes are needed to ensure there is appropriate consent throughout the study, including in circumstances where a participant might lose capacity during their involvement in the study. The Research Conduct page of the website has more information on this.
- Given by a person who has capacity
- Given voluntarily with no undue influence
- Given by someone who has been adequately informed
- Represent that person's fair choice
Although most adults are able to make decisions for themselves (and there is a presumption under the Mental Capacity Act that adults are able to unless proven otherwise), there are some adults who will not have capacity to do so. The Communication and Capacity page of the website has more information on assessing capacity for research and how to ensure that the person is informed and supported in order to maximise their ability to their own decision where possible.
There are additional legal and ethical considerations for adults who lack capacity to be included in research. More information can be found on the Legal Frameworks page and Research Ethics page.
Consent is considered to be an iterative and on-going process rather than a one-off event. Researchers will need to consider what processes are needed to ensure there is appropriate consent throughout the study, including in circumstances where a participant might lose capacity during their involvement in the study. The Research Conduct page of the website has more information on this.
Consent arrangements for adults who lack capacity to consent
Adults who are unable to consent for themselves should be included in research, provided that the arrangements for their inclusion are in accordance with the relevant legal frameworks and ethical principles. In this context, an adult is anyone aged 16 or over.
The consent arrangements for research involving adults who are unable to consent for themselves depends on the type of study and where in the UK the research is taking place. These arrangements include who is involved in making a decision about enrolment on behalf of the person, the legal basis for their decision, and whether they are being asked to provide consent on behalf of the person or are consulted for their advice. More information can be found on the Legal Frameworks page and on the Research Ethics page, including a map by the Health Research Authority which outlines the relevant legislation in the UK.
Our previous study explored how consultees and legal representatives made decisions about research on behalf of someone who lacked capacity to consent.
The consent arrangements for research involving adults who are unable to consent for themselves depends on the type of study and where in the UK the research is taking place. These arrangements include who is involved in making a decision about enrolment on behalf of the person, the legal basis for their decision, and whether they are being asked to provide consent on behalf of the person or are consulted for their advice. More information can be found on the Legal Frameworks page and on the Research Ethics page, including a map by the Health Research Authority which outlines the relevant legislation in the UK.
Our previous study explored how consultees and legal representatives made decisions about research on behalf of someone who lacked capacity to consent.
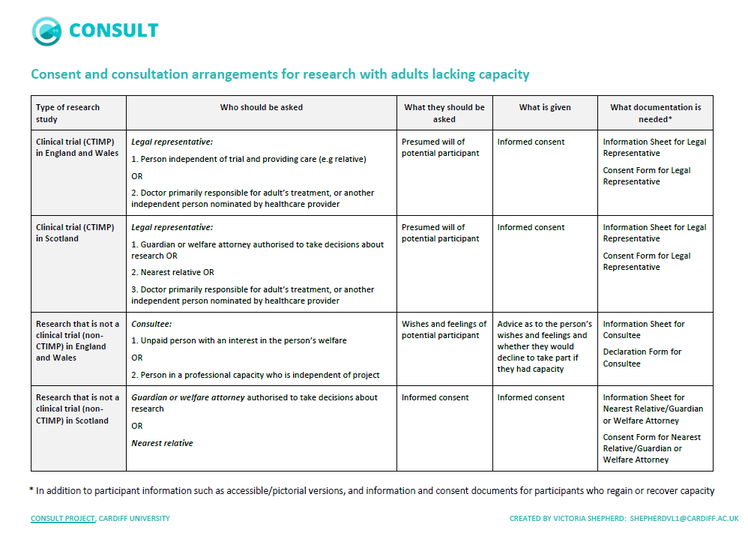
Summary of consent and consultation arrangements
This summary outlines who is involved in decisions about participation, what they should be asked, and what is given in terms of providing consent or other legal arrangements.
Researchers should also bear in mind any additional language requirements including translation, and ensuring that information about research is culturally appropriate and avoids discrimination (see the INCLUDE Ethnicity Framework on the Trial Forge website).
Role of 'assent' for adults lacking capacity
Individual jurisdictions may use the term assent in a range of different contexts. However, in the UK the term assent is considered to only apply to research involving children and not adults lacking capacity - it is not included in the Mental Capacity Act or clinical trials legislation - although it is sometimes used informally to describe the process of involving or informing the person who lacks capacity. Adults who lack capacity to consent should be informed, involved, and supported to make decisions at all stages of research, however there is no legal basis for requiring 'assent' to be obtained or to require the completion of ‘assent forms’ or similar processes.
The legal requirement to provide information about a study to an adult who lacks capacity to consent, and the legal weight that must be given to any dissent or objection, varies depending on the type of research and where in the UK it is conducted. There is more information in the summary table on the Legal Frameworks page.
The term assent is sometimes erroneously used in the UK to describe the agreement obtained from either a consultee or legal representative on behalf of the person who lacks capacity. However, depending on the study type, they either provide advice or consent.
The legal requirement to provide information about a study to an adult who lacks capacity to consent, and the legal weight that must be given to any dissent or objection, varies depending on the type of research and where in the UK it is conducted. There is more information in the summary table on the Legal Frameworks page.
The term assent is sometimes erroneously used in the UK to describe the agreement obtained from either a consultee or legal representative on behalf of the person who lacks capacity. However, depending on the study type, they either provide advice or consent.
Role of advance decisions and power of attorney
Nothing must be done in the course of the research which would be contrary to any advance decision made by the person (Mental Capacity Act s33). There is uncertainty about the role of an Advance Decision to Refuse Treatment (ADRT) in decisions about research under the MCA. An ADRT, which is legally binding, would be considered to apply to any specific treatment that forms part of a research study and so must be taken into account by consultees and legal representatives. However, the MCA does not have any provisions about an ADRT applying to research participation. Other forms of non-legally binding decisions such as advance statements should also be considered by consultees and legal representatives.
Under the MCA, a person can register for someone to have Lasting Power of Attorney (LPA) over their affairs prior to becoming incapacitated. This can include a Health and Welfare LPA who can make decisions about their personal welfare at a point when they lack capacity, including healthcare decisions and consent to medical treatment. Detailed guidance on the role of the attorney (or 'donee') is set out in chapter 7 of the MCA Code of Practice.
A family member or friend does not have to hold LPA in order to act as a personal consultee or legal representative. Someone authorised under a registered Lasting Power of Attorney or who is a deputy appointed by the Court of Protection can act as a consultee or legal representative, provided that they are not acting in a professional or paid capacity (e.g if they were the person’s solicitor). However, in decisions about research someone holding LPA would either be providing advice as a consultee or giving consent as a legal representative. This is unlike for treatment decisions where they would be providing consent as an LPA.
Encouraging people to have early discussions about their research preferences when appointing a Health and Welfare LPA may be useful.
Under the MCA, a person can register for someone to have Lasting Power of Attorney (LPA) over their affairs prior to becoming incapacitated. This can include a Health and Welfare LPA who can make decisions about their personal welfare at a point when they lack capacity, including healthcare decisions and consent to medical treatment. Detailed guidance on the role of the attorney (or 'donee') is set out in chapter 7 of the MCA Code of Practice.
A family member or friend does not have to hold LPA in order to act as a personal consultee or legal representative. Someone authorised under a registered Lasting Power of Attorney or who is a deputy appointed by the Court of Protection can act as a consultee or legal representative, provided that they are not acting in a professional or paid capacity (e.g if they were the person’s solicitor). However, in decisions about research someone holding LPA would either be providing advice as a consultee or giving consent as a legal representative. This is unlike for treatment decisions where they would be providing consent as an LPA.
Encouraging people to have early discussions about their research preferences when appointing a Health and Welfare LPA may be useful.
Consent and consultation resources
These resources are intended to support consent and consultation processes for research involving adults with impaired capacity and to assist researchers when designing associated documentation.
British Psychological Society guidanceConducting research with people not having the capacity to consent to their participation: A practical guide for researchers
|
Participant document templatesTemplates for accessible participant information sheets and consent forms from the Collaboration of Aphasia Trialists (CATs)
|
HRA examples of documentsExample consultee information sheets and declaration forms in England and Wales, and for Legal Representatives in Scotland
|
Consent Support ToolTool to facilitate involvement of people with communication disorders in health research studies, and to help health research professionals obtain informed consent from this population
|
Easy read image bank'Easy on the i' image bank of symbols, photos and graphics with images both in box and also out of the box ready to download
|
Accessibility resourcesRange of resources to help make information accessible for people with learning disabilities
|
Consent and recruitment in ICU researchResources from the Perspectives Study (Perspectives on enhancing consent and recruitment in intensive care studies) including an animation of the findings and guidance documents
|
Consent with people with HDGaining and maintaining consent when capacity can be an issue with people with Huntington’s disease, including a description of the development of written information adapted according to the needs of potential participants
|
eConsent with people with dementiaPaper exploring the eConsent process for people living with dementia, including the applicability of this process for people with cognitive impairment, their data privacy rights, and whether their needs and those of their caregivers have been neglected
|
Patient video on 'deferred consent'An animated video created for adult patients who have been enrolled in emergency research explaining about research without prior consent (or 'deferred consent')
|